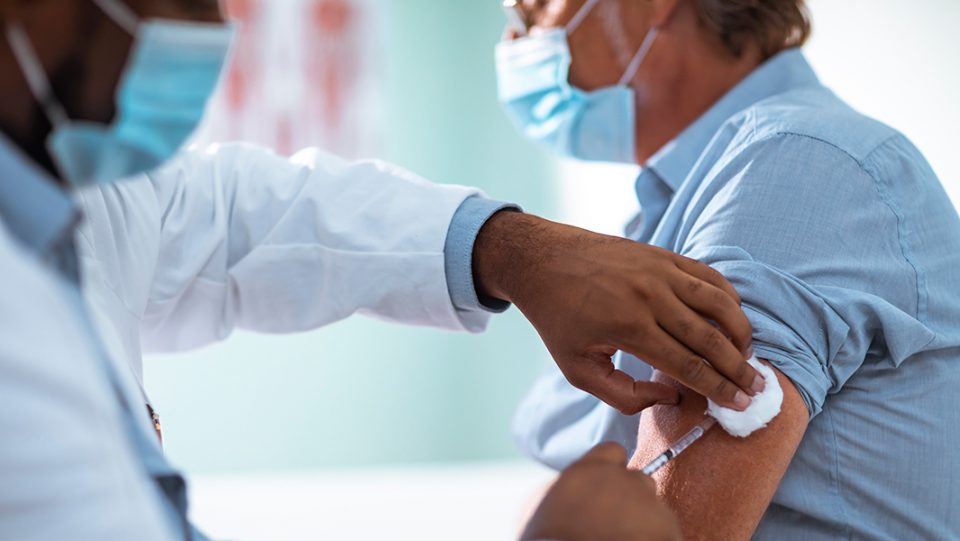
More clinical and real-world data is needed on how well and for how long COVID-19 vaccines are protective before any decisions should be made on offering a third or booster doses, Europe’s drug regulators said on Wednesday. Speaking at a news briefing on the coronavirus pandemic, the European Medicines Agency’s head of biological health threats and vaccines strategy Marco Cavaleri cautioned against making “premature” moves to deploy booster COVID-19 shots.
“We need to look into real-world evidence to give us the data we need to know when would be the right time to give the third dose. We need to have data that show in the field, either real-world evidence or clinical trials, that show what is the level of protection that is retained by the vaccines that we currently have,” he said.
- L&T Shares Trade With Nominal Losses Despite Securing Orders
- Daily vs Weekly SIP: Which Can Supercharge Your Portfolio to the Million-Mark?
- Rail Vikas Nigam Shares Take a Hit Despite Rs 165 Crore Order
- Lupin Shares Gain 1% as Unit Receives EIR Status from USFDA
- Glenmark Unit Signs Exclusive NSCLC Drug Pact with Hansoh Pharma; Shares Drop 1%
The European Union has already begun ordering COVID-19 booster vaccines and Britain and the United States have also begun preparing plans to offer third doses before the winter. Asked about evidence so far on COVID-19 vaccine performance against a new variant that emerged in India, Cavaleri said EMA was monitoring the situation “very closely” and that data was “rather reassuring” for vaccines in use in the European Union.
 Live
Live