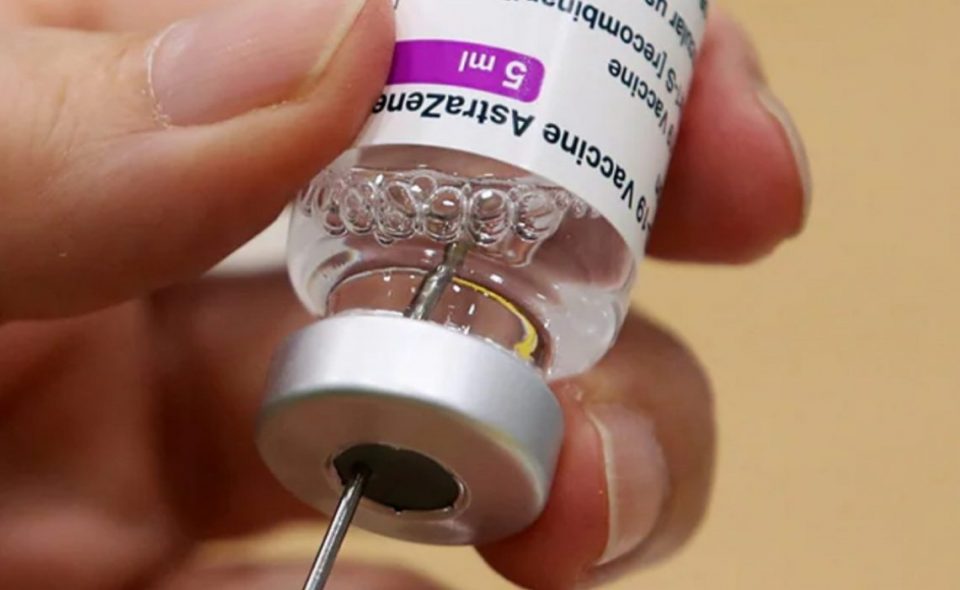
AstraZeneca said its COVID-19 vaccine was 76 per cent effective at preventing symptomatic illness in a new analysis of its major US trial a tad lower than the level announced earlier this week in a report that was criticised for using outdated information. US health officials had publicly rebuked the drugmaker for not using the most up-to-date information when it published an interim analysis on Monday that said the vaccine was 79 percent effective.
The latest data was based on 190 infections among more than 32,400 participants in the United States, Chile, and Peru. The earlier interim data was based on 141 infections through February 17. “The primary analysis is consistent with our previously released interim analysis, and confirms that our COVID-19 vaccine is highly effective in adults,” Mene Pangalos, executive vice president of BioPharmaceuticals R&D at AstraZeneca said in a statement.
- Welspun Corp Bags Rs 2,400 Crore Coated Pipe Orders in the US, Shares Trade Flat
- Zydus Life Gets USFDA Nod for Eluxadoline Tablets, Shares Up 1.5%
- HCC-Tata Projects JV Wins Rs 2,191 Crore Indore Metro Contract, Shares Up 2.5%
- Stocks in Focus: GR Infraprojects, KEC International, Shilpa Medicare, and Others
- Stocks Under F&O Ban: Steel Authority of India, Hindustan Copper, and Others
AstraZeneca said it plans to seek US emergency use authorisation in the coming weeks and the latest data has been presented to the independent trial oversight committee, the Data Safety Monitoring Board. AstraZeneca reiterated on Thursday that the shot, developed with Oxford University, was 100 per cent effective against severe or critical forms of the disease. It also said the vaccine showed 85 percent efficacy in adults 65 years and older.