Cipla Ltd shares have gained as much as 16 per cent after the company received approval from the US Food and Drug Administration (USFDA) for its generic version of Proventil HFA Inhalation Aerosol drug.
At 10.45 am, the stock traded at Rs 585.60, up 14.15 per cent from its previous close, while the benchmark Sensex rose 2.93 per cent and BSE Healthcare index advanced 4.74 per cent.
In an exchange filing, Cipla said it has received ‘final approval for its abbreviated new drug application (ANDA) for Albuterol Sulfate Inhalation Aerosol 90mcg (base)/actuation from the USFDA.’ The drug is the first AB-rated generic therapeutic equivalent version of Merck Sharp & Dohme Corp’s Proventil HFA Inhalation Aerosol. It is used to treat acute episodes of bronchospasm or prevention of asthmatic symptoms. According to IQVIA (IMS Health), Proventil and its authorised generic equivalent had sales of approximately $153 million in the US for the 12 months ended February 2020.
The entire Albuterol Sulfate HFA Inhalation Aerosol market had US sales of approximately $2.8 billion during the same period. ‘We are pleased to receive the final approval for generic Albuterol MDI from the USFDA. This further strengthens our presence in the US market. Albuterol is the first generic metered dose inhaler of Proventil HFA Inhalation Aerosol ever approved by FDA in the US and Cipla’s first device-based inhalation product in the market,’ said Umang Vohra, managing director and chief executive officer (MD & CEO), Cipla.
Read EquityPandit’s Cipla Outlook for the Week
Raju Srivastava Passes Away in Delhi

On Wednesday, Raju Srivastava, the famous comedian, passed away at the age of 58 years. He was admitted to the intensive care unit (ICU) of AIIMS on August 10. When he was running on the treadmill, he complained about chest pain. He underwent an angioplasty the same day. Recently the doctors have changed the pipe of Raju Srivastava’s ventilator to avert infection. At the same time, due to infection, his wife Shikha and daughter Antara were also not allowed to meet him. He lived with his wife Shikha and two children, Antara and Ayushman.
- Govt to impose 10% import duty on desi chana from 1st April
- Xi Jinping Meets Global CEOs Amid Slowing Investment
- ChatGPT’s Viral Ghibli-Style Images Spark AI Copyright Debate
- BEML Shares Extend Gains on Rs 405 Crore Contract
- Force Motors Shares Soar 7% on Securing Order for Force Gurkha Light
“I got a call from the family about half an hour ago saying he is no more. It is really unfortunate news. He was fighting in the hospital for over 40 days,” his brother Dipoo Srivastava told PTI.
Raju Srivastava was declared dead at 10.20 am, according to hospital sources.
Defence Minister Rajnath Singh extended condolences on the demise of comedian Srivastava. “Apart from being an accomplished artist, he was also a very lively person,” the minister wrote.
Taking to Twitter, Rajnath Singh added, “I am deeply saddened by the passing away of renowned comedian, Raju Srivastava Ji. Apart from being an accomplished artist, he was also a very lively person. He was also very active in the social field. I extend my condolences to his bereaved family and fans. Shanti!”.
Srivastava catapulted to fame after participating in the first season of the reality stand-up comedy show “The Great Indian Laughter Challenge” in 2005.
Srivastava has also featured in Hindi films such as “Maine Pyar Kiya”, “Baazigar”, the remake of “Bombay to Goa”, and “Aamdani Atthani Kharcha Rupaiya”. He is the chairperson of the Film Development Council of Uttar Pradesh.
He also joined Bhartiya Janta Party on 19 March 2014. Prime Minister Narendra Modi nominated him to be part of the Swachh Bharat Abhiyan.
SC Sentences Vijay Mallya to 4 Months Jail in Contempt of Court Case

The Supreme Court sentenced Vijay Mallya, declared a fugitive in a 2017 contempt of court case, to four months in prison on July 11. The court also imposed a fine of Rs 2,000, payable within four weeks.
Mallya paid her family $40 million in violation of a court order. For this, he was found guilty of contempt of court. The Supreme Court noted that despite being held in contempt of court, Mallya has never shown remorse or apology.
In a significant move, the Supreme Court has directed the deal to be reversed for $40 million and required to deposit the funds at 8% interest over four weeks.
In March, the Supreme Court reserved its order on the number of penalties in Mallya’s contempt of court case.
On a related note, Mallya did not comment on the amount of the Supreme Court penalty, despite giving him the opportunity.
The court had earlier postponed the case to give Mallya time to present his case to the court in person or through a lawyer. However, Mallya neither appeared in court nor was instructed by a lawyer.
So, the Supreme Court went ahead with the case and upheld its ruling after a lengthy amicus curiae hearing. The Supreme Court has appointed senior defence lawyer Jaideep Gupta to assist in the case as amicus curiae, which means friend of the court.
Mallya was given another opportunity to present his case through a written submission.
Today’s verdict relates to a 2017 Supreme Court decision in which Vijay Mallya was held in contempt of court for willfully violating the court order. The Supreme Court affirmed the contempt of court conviction while denying Mallya’s request to review the ruling.
Mallya was instructed to appear in court for a hearing on the amount of the fine, but he was still unable to attend due to the pending extradition proceedings against him in the UK at the time.
Following the extradition process, part of the process, the nature of which is still unknown to Indian authorities, is still ongoing in the UK.
UK Court Hears Nirav Modi’s Plea Against Extradition to India

On Tuesday, a UK court opened a continuation appeal hearing in the extradition case of Nirav Modi, who is wanted in India on charges of fraud and money laundering in the Punjab National Bank (PNB) loan fraud case worth an estimated $2 billion.
The 51-year-old diamond merchant appealed his extradition order last year on mental health grounds. Justices Jeremy Stewart-Smith and Robert Jay presided over preliminary hearings in the High Court in December to determine whether District Judge Sam Goozee’s Westminster Magistrate ruling in favour of extradition from February 2021 was incorrect, ignoring the “high risk of suicide” for Nirav Modi.
This week’s hearing is to continue the appeal, and a verdict could be expected soon. Suppose Nirav Modi wins his appeal hearing in the High Court. In that case, he cannot be extradited unless the Indian government successfully obtains leave to appeal in the Supreme Court on issues of law of public importance.
On the other hand, if he loses this appeal hearing, Nirav Modi can bring a legal issue of public importance to the Supreme Court within 14 days of the High Court’s decision to appeal the High Court’s decision to the Supreme Court appeal.
However, this involves a high threshold, as an appeal to the Supreme Court can only be made if the High Court proves that the case involves a question of law of general public importance.
Finally, after all, avenues in the UK courts have been exhausted, he can still seek a so-called Article 39 injunction from the European Court of Human Rights (ECHR).
According to officials familiar with the case, the Indian government has given assurances about the conditions under which Nirav Modi will be detained after he surrenders to India and the facilities available to care for his “physical and mental health”. The parties will now decide on the adequacy and credibility of these assurances.
“He is already at high risk of suicide, and his condition may deteriorate further in Mumbai,” Edward Fitzgerald QC argued on behalf of Modi at an appeal hearing in December.
Meanwhile, Nirav Modi has been held at Wandsworth Prison in southwest London since his arrest in March 2019.
Nirav Modi is the subject of two criminal proceedings, the CBI case involving large-scale fraud against PNB through a fraudulent obtaining of Letter of Undertakings (LoUs) or loan agreements, and the ED case involving money laundering of the proceeds of the fraud.
He also faces two other charges of “causing evidence to disappear” and intimidating a witness or “criminal intimidation leading to death,” which were added to the CBI case.
LinkedIn, UN Women Join Forces to Create Jobs for Women

LinkedIn, the world’s largest professional network, said on Wednesday it would invest $500,000 or Rs 3.88 crore in partnership with UN Women to promote women’s economic empowerment.
The statement said that the project would be piloted in Maharashtra to develop digital, soft and employable skills for 2,000 women and provide them with career development opportunities through job fairs, mentoring sessions and peer-to-peer networking.
It added that the three-year regional cooperation would digitally upskill women, provide them with more employment opportunities, and enable them to participate in the formal economy fully.
The partnership will follow the Women’s Empowerment Principles (WEP), a set of effective, actionable principles on how businesses can promote gender equality and women’s empowerment in the workplace, markets and communities.
UN Women and LinkedIn will leverage online platforms and institutional expertise to convene partners to support women’s economic empowerment.
Together, they will host joint advocacy campaigns and events and bring key partners from their respective networks to achieve broadly equal opportunities and outcomes for women and men in the workplace.
In addition, UN Women will use its partnership to provide young women with onboarding opportunities across industries, focusing on operating the LinkedIn platform and network.
A disproportionate number of women lack basic internet access. The statement said that 54.6% of men in Asia have access to the internet, compared to 41.3% of women. Hence, developing gender-sensitive technology policies in the Asia-Pacific region is crucial.
In an increasingly digital world, women and girls often do not have access to the same educational opportunities or types of education as men and boys, which sometimes reduces their digital skills and literacy rates, leading to reduced economic opportunities. The massive impact of COVID-19 over the past two years has only widened the opportunity gap for women and girls.
- Govt to impose 10% import duty on desi chana from 1st April
- Xi Jinping Meets Global CEOs Amid Slowing Investment
- ChatGPT’s Viral Ghibli-Style Images Spark AI Copyright Debate
- BEML Shares Extend Gains on Rs 405 Crore Contract
- Force Motors Shares Soar 7% on Securing Order for Force Gurkha Light
After a 15-month pilot, UN Women and LinkedIn will integrate lessons learned and assessment feedback as needed to refine the program before rolling it out to other Asia-Pacific countries where UN Women is located.
Moosewala Murder: First Police Arrest Cousin of Jailed Gangster
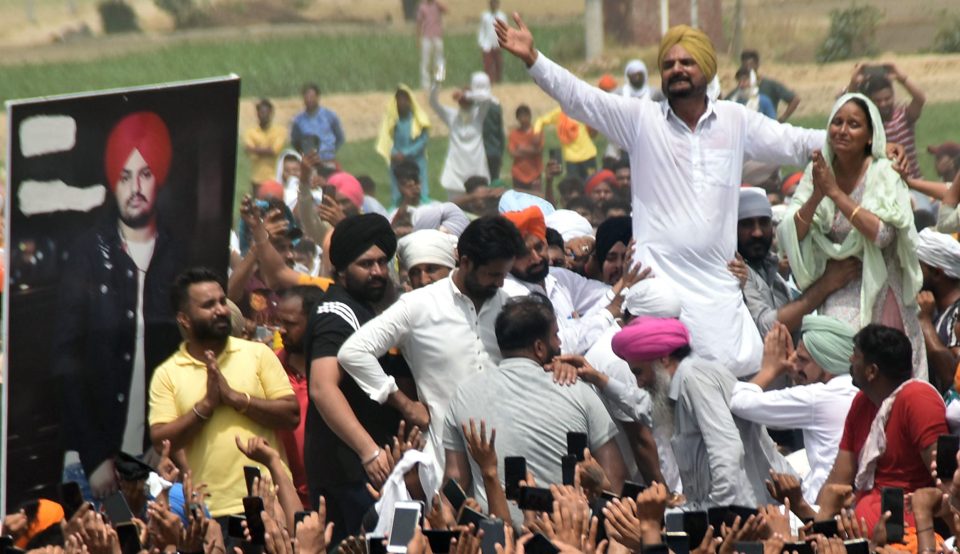
Punjab police on Tuesday arrested a man in connection with the brutal murder of singer-politician Sidhu Moosewala.
The accused, Manpreet Singh Bhau, aged 27, is a resident of Dhapyi village in the Faridkot district. Manpreet is accused of providing vehicles to the killers involved in Moosewala’s murder. According to police, Manpreet was not one of the killers who indiscriminately shot Moosewala in his car and killed him on the spot.
Manpreet is the cousin of another gangster Manpreet Singh Manna, a prisoner in Ferozepur jail and was brought to Mansa on a production warrant for questioning in the Moosewala murder case on Monday. Manna is in jail for killing another gangster, Kulbir Naruana.
A white Toyota Corola owned by Manpreet Manna has been handed over to the attackers, police said. CCTV footage on social media showed a white car behind Moosewala’s car in the village of Jawaharke on Sunday evening. The occupants of the vehicle first fired at Moosewala’s vehicle. Later another group of assassins, a Mahindra Bolero car, joined the first group and started firing indiscriminately; Moosewala was killed on the spot.
There are nine cases against Manpreet in Faridkot, Ropar and Muktsar under the Arms Act and nine cases of kidnapping and murder. On Monday, he was among six people arrested from Dehradun while returning from Hemkund Sahib, a Sikh temple in Uttarakhand’s Chamoli district. They were later brought to Punjab for questioning in the Moosewala murder case.
Manpreet was produced in Mansa court, where he was remanded in police custody for five days. Police are expecting more information from Manpreet about the attackers. Moosewala was shot dead by unidentified assailants in Punjab’s Mansa district a day after the state government withdrew his security cover.
State police called the incident an inter-gang conflict and said the Lawrence Bishnoi gang was behind the killings. Goldy Brar, a Canadian-based gangster who is a member of the Bishnoi gang, has claimed responsibility for the killings.
According to police, Moosewala’s assassination appears to have been retaliation for last year’s assassination of young Akali leader Vicky Middukhera. Moosewala’s manager Shagunpreet was named in the Midukhera murder case. However, police said that Shagunpreet had fled to Australia.
- Govt to impose 10% import duty on desi chana from 1st April
- Xi Jinping Meets Global CEOs Amid Slowing Investment
- ChatGPT’s Viral Ghibli-Style Images Spark AI Copyright Debate
- BEML Shares Extend Gains on Rs 405 Crore Contract
- Force Motors Shares Soar 7% on Securing Order for Force Gurkha Light
The three shooters, identified as Sunny, Anil Lath and Bhalu, were arrested by a special cell of Delhi Police in connection with the murder of Haryana resident Vicky Midukhera and Shagunpreet was also named as an accused in the FIR.






