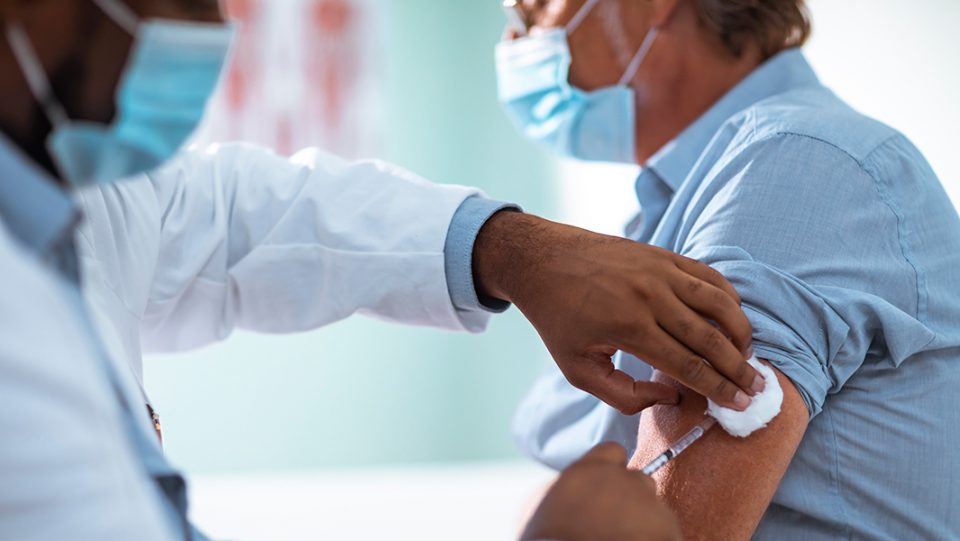
More clinical and real-world data is needed on how well and for how long COVID-19 vaccines are protective before any decisions should be made on offering a third or booster doses, Europe’s drug regulators said on Wednesday. Speaking at a news briefing on the coronavirus pandemic, the European Medicines Agency’s head of biological health threats and vaccines strategy Marco Cavaleri cautioned against making “premature” moves to deploy booster COVID-19 shots.
“We need to look into real-world evidence to give us the data we need to know when would be the right time to give the third dose. We need to have data that show in the field, either real-world evidence or clinical trials, that show what is the level of protection that is retained by the vaccines that we currently have,” he said.
- Trump-backed GOP Funding Plan Fails in House as Shutdown Looms
- Italy Fines OpenAI 15 Million Euros for Privacy Breach
- India’s Forex Reserves Drop to $652.87 Billion as of 13th Dec
- World Bank Approves $800 Mn Loan for Amaravati Project
- How Mutual Funds Work? Types Of Mutual Funds, Tax And Example Of Mutual Funds
The European Union has already begun ordering COVID-19 booster vaccines and Britain and the United States have also begun preparing plans to offer third doses before the winter. Asked about evidence so far on COVID-19 vaccine performance against a new variant that emerged in India, Cavaleri said EMA was monitoring the situation “very closely” and that data was “rather reassuring” for vaccines in use in the European Union.